
The NPRU participates in numerous clinical trials and research studies each year to search for new and better ways to care for our babies and improve their health outcomes. Current research studies that focus on Congenital Diaphragmatic Hernia (CDH) are outlined below.
Overview
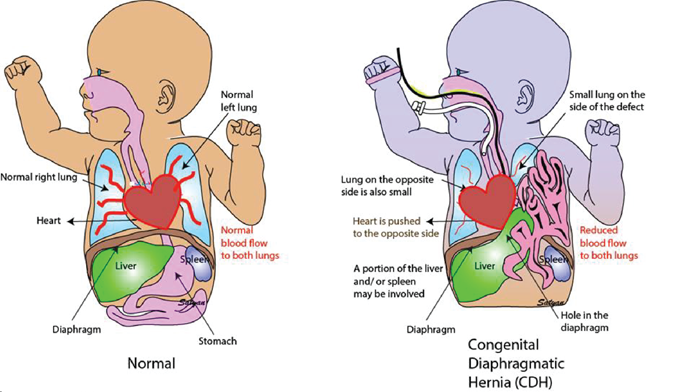
Congenital Diaphragmatic Hernia (CDH) is a result from the incomplete formation of the diaphragm, the muscle that separates the abdomen from the chest. The resulting hole is either on the left or the right side (more commonly the left) and may be small and allow a limited amount of intestine into the chest, or it may be large and allow a very large amount of abdominal contents to herniate from the abdomen into the chest, starting early in development. The resulting mass of abdominal organs in the chest, which can include intestine, stomach, spleen and even liver, takes necessary space away from the developing lungs. The result is small lungs, varying from moderately to severely small, and making it hard for the baby to breathe. A larger hole creates a larger impact on the lung size, so CDH presents as a spectrum of severity from less severe to highly severe.
CDH is repairable, and occurs in about 1 in every 2,500 live births in the US every year. It is most often diagnosed before birth. Immediately after birth, infants born with CDH will be stabilized in the intensive care nursery. Typically, surgical repair occurs several days after delivery. The cause of CDH is unknown.
Milrinone in Congential Diaphragmatic Hernia (CDH)
This is a double-blind randomized controlled pilot study examining the use of milrinone in diaphragmatic hernia for infants with a prenatal or postnatal diagnosis of CDH. This initial pilot study will help us to understand how milrinone works to help the heart and lungs give oxygen to the tissues and other organs in infants with CDH. Milrinone is a medication that is currently approved by the Food and Drug Administration (FDA) for short-term use in adults with heart failure. We are hoping this pilot study will support and look at the possibility and safety of conducting a much larger study.
An Observational Study of Infants with Congenital Diaphragmatic Hernia (CDH)
This is a multicenter/multinational, observational registry of children born with CDH. Since inception in 1995, over 3500 children have been entered, from 90 centers represented by 10 countries around the world. The purpose of this Quality Improvement (QI) registry and the CDH Study Group is to collect and analyze information on CDH with the hope that with careful delineation of the natural history of this disease the information will lead to identifying appropriate interventions that will directly benefit infants born with CDH.

Duke’s Department of Neonatology is part of the NICHD, a collaborative network of researchers devoted to improving neonatal and maternal outcomes. Depending on the severity of your baby’s CDH, he or she may have the opportunity to participate in a clinical research study. Participation in any research study is voluntary and will not impact the care your baby receives.
Samia Aleem, MD, MHS, Duke Principal Investigator and Neonatologist
How you can help
Our mission is to provide the professional infrastructure and clinical expertise directed toward improving the quality of care and long-term outcomes for our babies. Find out how you can help support our babies and our research.
How you can participate
If you currently have a baby in the Duke Intensive Care Nursery, and you think you may want to participate in any of our research studies, please ask your baby’s nurse or doctor to contact us. The NPRU team will then contact you to explain the criteria for study eligibility and describe the potential benefits and risks for entering the study. Learn about our current research opportunities.
Learn more
To learn more about the Duke Intensive Care Nursery, visit the Duke Division of Neonatology website. For more information about the Neonatal Perinatal Research Unit or to learn more about our clinical trials, email us at npru@duke.edu, or call 919-681-4913.